Formulation Development Outsourcing Market
Formulation Development Outsourcing Market Overview
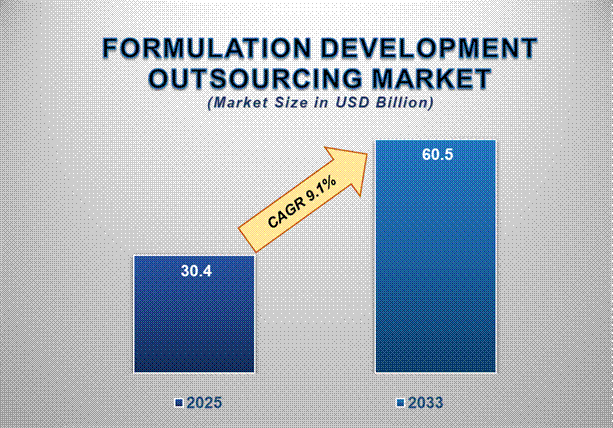
The Formulation Development Outsourcing Market size is projected to witness significant growth from 2025 to 2033, driven by the rising demand for new drug development. Valued at approximately USD 30.4 billion in 2025, the market is expected to reach USD 60.5 billion by 2033, reflecting a strong compound annual growth rate (CAGR) of 9.1% over the forecast period.
Get Report Link https://m2squareconsultancy.com/reports/formulation-development-outsourcing-market
Market snapshot
Analysts put the global formulation development outsourcing market in the tens of billions today and project steady mid-single-digit to high-single-digit growth through the coming decade. Recent reports estimate market sizes in the mid-to-high $20–40 billion range for the mid-2020s, with compound annual growth rates commonly reported between about 5% and 7% (and some forecasts slightly higher depending on scope and definition).
Why demand is rising
Several clear drivers explain why formulation outsourcing has accelerated:
-
R&D cost pressure and efficiency needs. Drug discovery budgets are large and unpredictable; outsourcing formulation lets sponsors convert fixed costs into variable spend and tap expertise only when needed.
-
-
Specialized expertise and technology platforms. Advanced delivery systems (controlled-release, inhalation, sterile injectables, lipid nanoparticles for mRNA, antibody-drug conjugates) require equipment and know-how many sponsors lack. Outsourcers invest in these capital-intensive capabilities and amortize them across clients.
-
-
Faster timelines and regulatory complexity. Regulators expect robust stability and compatibility data. CDMOs and formulation specialists streamline development paths and provide regulatory packages that de-risk filings.
-
-
Biologics and personalized medicine. Growth in biologics, cell & gene therapies, and niche oncology compounds increases demand for small-batch, high-complexity formulation and fill/finish services.
-
Key trends shaping the market
-
Platform diversification. Providers are expanding beyond traditional oral solid dose into injectables, sterile manufacturing, inhalation, transdermal, and complex parenteral platforms to win larger program work.
-
-
Integration of digital tools and AI. Machine learning and predictive analytics are being applied to optimize formulations, accelerate stability modeling, and reduce experimental cycles — improving speed and lowering cost.
-
-
Geographic rebalancing and nearshoring. Sponsors are diversifying supply chains and seeking capacity closer to end markets. That’s driving expansion of CDMO/formulation footprints in North America, Europe, and rapidly in Asia-Pacific. Recent deals and acquisitions show Indian and other CRO/CDMO players actively expanding capabilities and cross-border presence.
-
-
End-to-end offerings. Larger CDMOs increasingly offer cradle-to-commercial services — from early formulation and analytical method development through clinical manufacturing and commercial supply — to capture more program value and provide single-vendor convenience.
-
Get Sample Report Link https://m2squareconsultancy.com/request-sample/formulation-development-outsourcing-market
Buy Now https://m2squareconsultancy.com/purchase/126
Regional outlook
North America and Europe remain dominant by spend and technology intensity, but Asia-Pacific (especially India and China) is growing fast as local CROs and CDMOs expand into higher-complexity services. Cost competitiveness plus deepening scientific talent pools make the region attractive for both early-stage development and cost-sensitive late-stage manufacturing. Several recent M&A moves and facility investments underline this trend.
Challenges for buyers and providers
-
Quality and regulatory risk. Outsourcing transfers execution risk; sponsors must carefully vet partners’ quality systems and regulatory track record.
-
-
Capacity squeezes. As demand surges in specific platforms (sterile injectables, biologics), premium capacity can be constrained, leading to project delays or higher costs.
-
-
IP and data security. Sharing detailed formulation and clinical data with external partners raises intellectual property and confidentiality concerns that must be contractually and technically managed.
-
Opportunities and where smart money is going
-
Specialist niches. Companies that master complex modalities — lipid nanoparticles, ADCs, inhalation, sustained-release injectables — can command premium margins.
-
-
Analytical and stability services. High-quality analytical method development, accelerated stability prediction, and extractables/leachables expertise are sticky services that deepen client relationships.
-
-
Flexible, small-batch biologics. The rise of personalized and niche biologics creates demand for agile small-scale formulation and fill/finish services. Investments in flexible suites and single-use technologies pay off.
-
Who’s winning?
Large CDMOs with global footprints (Lonza, Catalent, Samsung Biologics, WuXi, Thermo Fisher and others) remain leaders because they combine scale, regulatory experience, and multi-platform capabilities. At the same time, mid-tier specialists that focus on oral solids, inhalation, sterile injectables, or niche delivery technologies carve defensible positions by investing in the right equipment and scientific talent.
Practical advice for sponsors
-
Match the partner to program risk: use small, nimble specialists for early formulation screening; move to a larger CDMO for clinical scale-up and commercial readiness.
-
-
Build strong technical transfer playbooks and joint quality governance so knowledge stays with the program even if teams or vendors change.
-
-
Insist on transparency in timelines, capacity commitments, and change-control procedures to avoid surprises as a program scales.
-
Conclusion
Formulation development outsourcing is no longer a cost-cutting afterthought — it’s a strategic channel for speed, capability access, and risk management. As drug modalities diversify and regulatory expectations rise, sponsors that pick the right mix of specialist and full-service partners will move faster from molecule to market. For providers, the winners will be those who invest in platform depth, regulatory excellence, and digital tools that compress development time without compromising quality.
Buy Now https://m2squareconsultancy.com/purchase/126
About m2squareconsultancy :
We are a purpose-driven market research and consulting company passionate about turning data into direction. Founded in 2023, we bring together researchers, strategists, and data scientists who believe that intelligence isn’t just about numbers, it’s about insight that sparks progress.
We cater to a wide range of industries by delivering customized solutions, strategic insights, and innovative support that help organizations grow, adapt, and lead in their respective sectors. Here’s a brief overview of key industries we work with
Contact Us:
Email: sales@m2squareconsultancy.com
Phone (IN): +91 80978 74280
Phone (US): +1 929 447 0100
https://m2squareconsultancy.com/reports/dental-software-market
https://m2squareconsultancy.com/reports/formulation-development-outsourcing-market
https://m2squareconsultancy.com/reports/healthcare-cloud-computing-market
https://m2squareconsultancy.com/reports/healthcare-predictive-analytics-market
https://m2squareconsultancy.com/reports/health-insurance-market
https://m2squareconsultancy.com/reports/heart-valve-devices-market
https://m2squareconsultancy.com/reports/global-ammunition-market
https://m2squareconsultancy.com/reports/smart-irrigation-market
https://m2squareconsultancy.com/reports/electrical-steel-market
https://m2squareconsultancy.com/reports/diagnostic-imaging-market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Juegos
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


