Asia-Pacific Clinical Trial Supplies Market Research Report: Market Size, Industry Statistics, and Forecast Analysis
"Future of Executive Summary Asia-Pacific Clinical Trial Supplies Market: Size and Share Dynamics
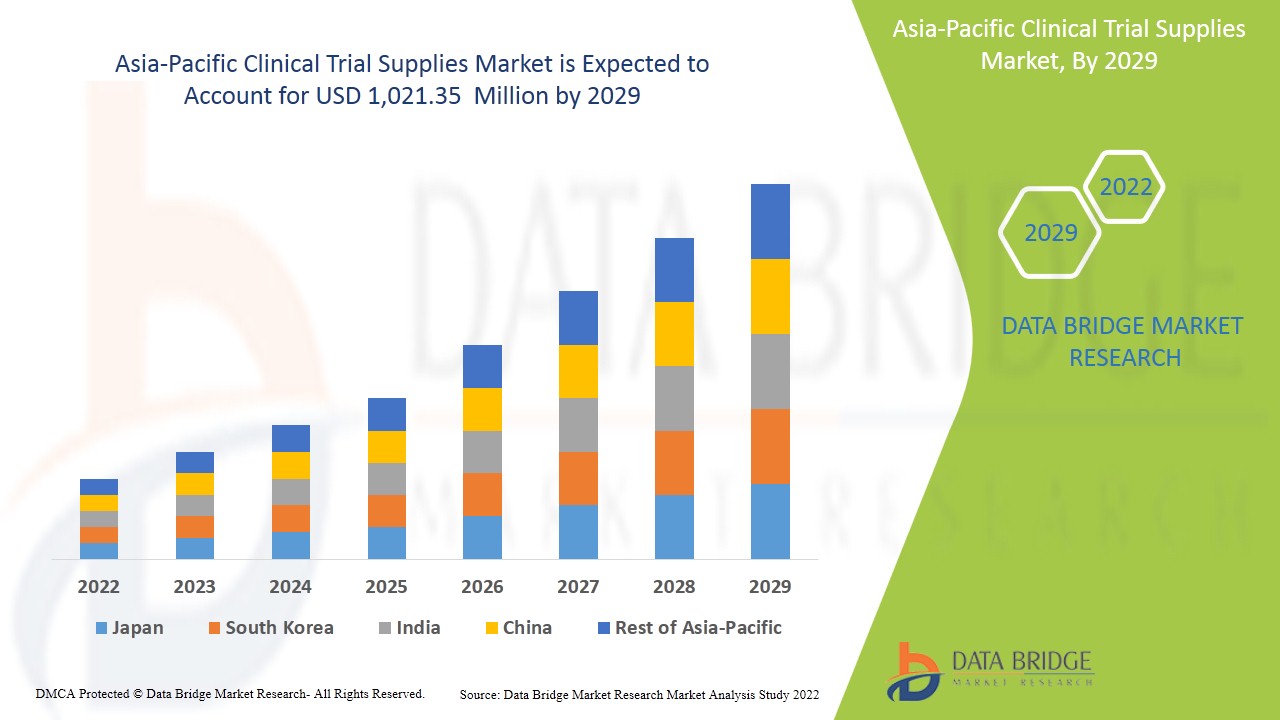
Data Bridge Market Research analyses that the market is growing with a CAGR of 9.2% in the forecast period of 2022 to 2029 and is expected to reach USD 1,021.35 million by 2029.
Businesses can attain detailed insights with the large scale Asia-Pacific Clinical Trial Supplies Market survey report which help them self-assuredly make decisions about their production and Market strategies in Asia-Pacific Clinical Trial Supplies Market industry. The report describes various parameters throughout which analyses the market status in detail. It also endows with statistics on the current state of the industry and hence works as a valuable source of guidance and direction for companies and investors interested in this market. The whole Asia-Pacific Clinical Trial Supplies Market report can be mainly categorised into four main areas which are market definition, market segmentation, competitive analysis and research methodology.
To have finest market insights and knowhow of the most excellent market opportunities into the specific markets, Asia-Pacific Clinical Trial Supplies Market research report is an ideal option. The report carries out the study of the market with respect to general market conditions, market status, market improvement, key developments, cost and profit of the specified market regions, position and comparative pricing between major players. Each topic of this report is examined very wisely to acquire a clear idea about all the factors that are influencing the market growth and Asia-Pacific Clinical Trial Supplies Market industry. The research study that has taken place in the large-scale Asia-Pacific Clinical Trial Supplies Market report covers the local, regional as well as global market.
Tap into future trends and opportunities shaping the Asia-Pacific Clinical Trial Supplies Market. Download the complete report:
https://www.databridgemarketresearch.com/reports/asia-pacific-clinical-trial-supplies-market
Asia-Pacific Clinical Trial Supplies Market Environment
Segments
- Product Type: The Asia-Pacific clinical trial supplies market can be segmented by product type into manufacturing & packaging services, clinical trial materials, and logistics & distribution services. The demand for manufacturing & packaging services is expected to rise due to the increasing emphasis on specialized services to support clinical trials.
- Phases: Based on phases, the market can be categorized into Phase I, Phase II, Phase III, and Phase IV clinical trials. With a growing number of Phase III and Phase IV trials being conducted in the region, there is a significant demand for clinical trial supplies during these phases.
- Therapeutic Area: Therapeutic areas such as oncology, cardiovascular diseases, infectious diseases, and others play a crucial role in driving the demand for clinical trial supplies in the Asia-Pacific region. The rising prevalence of chronic diseases fuels the need for conducting clinical trials in these therapeutic areas.
Market Players
- Thermo Fisher Scientific Inc.: One of the leading players in the Asia-Pacific clinical trial supplies market, Thermo Fisher Scientific offers a wide range of services including manufacturing, packaging, and distribution of clinical trial supplies.
- Catalent, Inc.: Catalent is another key player in the market known for its expertise in providing integrated services for clinical trials such as formulation development, packaging, and labeling.
- Sharp Packaging Services: Sharp Packaging Services is a prominent player offering cutting-edge solutions for clinical trial supplies management, including cold chain logistics and direct-to-patient distribution services.
The Asia-Pacific clinical trial supplies market is witnessing significant growth due to the increasing focus on research and development activities in the region. With a rising number of clinical trials being conducted across various therapeutic areas, the demand for high-quality clinical trial supplies and services is on the rise. Factors such as advancements in logistics and distribution services, growing investments in healthcare infrastructure, and favorable government initiatives are driving the market growth. The presence of key market players offering a wide range of services further contributes to the expansion of the clinical trial supplies market in the Asia-Pacific region.
The Asia-Pacific clinical trial supplies market is poised for robust growth in the coming years, driven by various factors that are shaping the landscape of clinical research in the region. One key trend that is expected to influence the market is the increasing focus on personalized medicine and precision therapies. As advancements in healthcare technology continue to evolve, there is a growing need for specialized clinical trial supplies to support these innovative treatment approaches. This shift towards personalized medicine is creating new opportunities for market players to develop tailored solutions that cater to specific patient populations and therapeutic areas, thereby driving the demand for customized clinical trial materials and services.
Moreover, the rise of contract research organizations (CROs) in the Asia-Pacific region is also contributing to the growth of the clinical trial supplies market. CROs play a vital role in supporting clinical trials by providing specialized services such as site management, patient recruitment, and data management. As the demand for outsourcing clinical trial activities continues to increase, CROs are partnering with clinical trial supplies companies to streamline the supply chain and ensure efficient delivery of materials to trial sites. This collaboration between CROs and suppliers is enabling greater flexibility and scalability in meeting the demands of complex clinical trials, thereby driving market growth.
Furthermore, regulatory reforms and harmonization efforts across the Asia-Pacific region are creating a more conducive environment for clinical research and drug development. With initiatives such as the ASEAN Common Technical Dossier (ACTD) and the Mutual Recognition Agreement (MRA) in place, there is greater alignment of regulatory standards and procedures, which simplifies the process of conducting multi-country clinical trials. This regulatory harmonization not only reduces the regulatory burden on sponsors but also enhances the efficiency and transparency of clinical trial operations, leading to accelerated timelines and improved data quality.
In conclusion, the Asia-Pacific clinical trial supplies market is positioned for dynamic growth, driven by trends such as personalized medicine, the emergence of CRO partnerships, and regulatory harmonization efforts. As the region continues to establish itself as a hub for clinical research and innovation, market players will need to adapt to these evolving trends and seize the opportunities presented by the changing landscape of healthcare. By leveraging technology, strategic partnerships, and a customer-centric approach, companies can differentiate themselves in the market and drive sustainable growth in the competitive clinical trial supplies sector in the Asia-Pacific region.The Asia-Pacific clinical trial supplies market is a dynamic and thriving sector driven by a multitude of factors that are reshaping the landscape of clinical research in the region. One of the primary drivers of market growth is the increasing focus on personalized medicine and precision therapies, which are revolutionizing the way healthcare treatments are developed and delivered. As advancements in healthcare technology continue to progress, the demand for specialized clinical trial supplies tailored to specific patient populations and therapeutic areas is expected to surge. This shift towards personalized medicine presents a significant opportunity for market players to innovate and develop customized solutions that address the evolving needs of the healthcare industry.
Additionally, the expanding presence of contract research organizations (CROs) in the Asia-Pacific region is playing a pivotal role in bolstering the clinical trial supplies market. CROs offer essential support for clinical trials by providing specialized services such as patient recruitment, site management, and data management. By collaborating with clinical trial supplies companies, CROs are optimizing the supply chain process and ensuring efficient delivery of materials to trial sites. This strategic partnership between CROs and suppliers enhances flexibility and scalability in addressing the requirements of complex clinical trials, thereby fueling market growth in the region.
Furthermore, regulatory reforms and harmonization initiatives across the Asia-Pacific region are creating a more conducive environment for clinical research and drug development. Efforts such as the ASEAN Common Technical Dossier (ACTD) and the Mutual Recognition Agreement (MRA) are aligning regulatory standards and procedures, simplifying the execution of multi-country clinical trials. This regulatory harmonization not only eases the regulatory burden on sponsors but also enhances the overall efficiency and transparency of clinical trial operations, resulting in accelerated timelines and improved data quality.
In conclusion, the Asia-Pacific clinical trial supplies market presents vast opportunities for growth and innovation, propelled by trends like personalized medicine, the increasing role of CRO partnerships, and regulatory harmonization efforts. To succeed in this competitive landscape, market players must stay abreast of evolving trends, leverage technological advancements, foster strategic collaborations, and prioritize a customer-centric approach. By embracing these strategies, companies operating in the clinical trial supplies sector can position themselves for sustainable growth and success in the burgeoning Asia-Pacific market.
Evaluate the company’s influence on the market
https://www.databridgemarketresearch.com/reports/asia-pacific-clinical-trial-supplies-market/companies
Forecast, Segmentation & Competitive Analysis Questions for Asia-Pacific Clinical Trial Supplies Market
- What is the estimated revenue size for the Asia-Pacific Clinical Trial Supplies Market?
- How fast is the Asia-Pacific Clinical Trial Supplies Market evolving?
- What are the emerging segments in this market?
- Who are the global influencers in the Asia-Pacific Clinical Trial Supplies Market?
- What are the breakthroughs in product development?
- What is the regional diversity in the Asia-Pacific Clinical Trial Supplies Market study?
- Which region is most attractive for new entrants?
- What countries are posting consistent growth?
- What markets are nearing saturation?
- What consumer behaviors are shaping future trends?
Browse More Reports:
North America Non-Surgical Procedures MarketEurope Residential Energy Management (REM) MarketAsia-Pacific Residential Energy Management (REM) MarketNorth America Residential Energy Management (REM) MarketAsia-Pacific Tunable Laser MarketEurope Tunable Laser MarketMiddle East and Africa Tunable Laser MarketNorth America Tunable Laser MarketAsia-Pacific Commercial Seaweed MarketMiddle East and Africa Commercial Seaweed MarketEurope Commercial Seaweed MarketGermany Wood Heating Stoves MarketAsia-Pacific Bio Surgical Agents MarketMiddle East and Africa Healthcare 3D Printing MarketNorth America Superhydrophobic Coating Market
Middle East and Africa Bulk Acoustic Wave Sensors Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness