Leukapheresis Devices Market Analysis: Strategic Insights, Revenue Projections, and Global Outlook to 2030
The global Leukapheresis Devices Market is currently experiencing a critical inflection point, transitioning from a niche therapeutic and donor-collection utility to a central component of the burgeoning cell and gene therapy ecosystem.
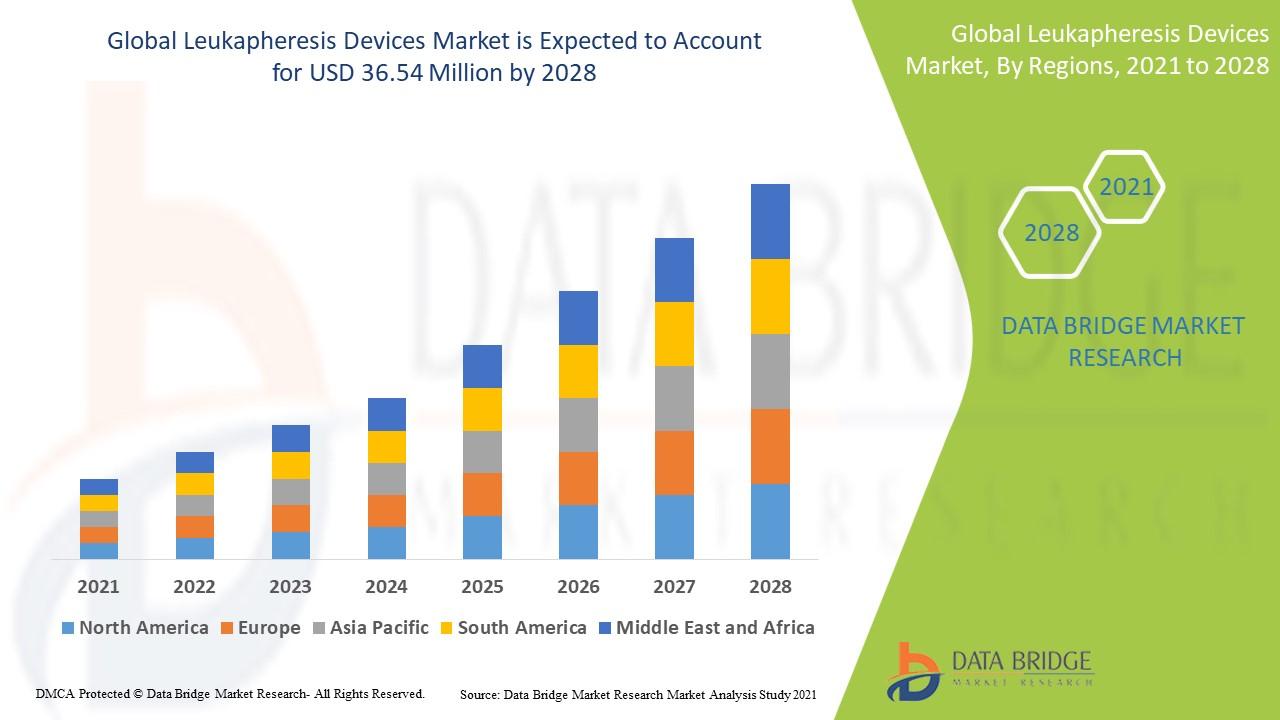
Data Bridge Market Research analyses that the leukapheresis devices market will exhibit a CAGR of around 41.18% for the forecast period of 2021-2028.
The procedure, which involves extracorporeal separation of white blood cells (leukocytes) from the blood, is now an indispensable upstream step for Chimeric Antigen Receptor (CAR-T) cell manufacturing. This powerful application is fundamentally reshaping the market’s revenue streams and competitive benchmarking.
The global Leukapheresis Devices Market, currently valued at $2.138 Billion, is poised for a transformative growth phase through 2030. The market is forecast to expand significantly due to the commercialization of advanced cell therapies, reaching a projected $4.651 Billion by 2035 at a robust Compound Annual Growth Rate (CAGR) of 7.32%.
- Critical Market Drivers:
- Rising global incidence of hematologic malignancies, such as leukemia and lymphoma.
- Exponential growth in research and clinical trials for T-cell-based immunotherapies (e.g., CAR-T).
- Technological advancements leading to highly automated and high-yield leukapheresis devices.
- Increasing demand for high-quality, standardized leukopaks for bio-pharma research and manufacturing.
- Favorable regulatory support and growing reimbursement for specialized apheresis procedures.
Market Landscape & Strategic Scope
The Leukapheresis Devices Market encompasses the entire value chain associated with the selective extraction of white blood cells. This includes the sophisticated automated devices (centrifugation-based and membrane separation systems), as well as the high-volume, recurring revenue stream generated by disposables (collection kits, tubing sets, and leukoreduction filters). The core value proposition of leukapheresis has evolved: while traditionally vital for managing hyperleukocytosis in acute leukemia patients and for collecting donor cells, its modern strategic scope is anchored by its essential role in the manufacturing pipeline for cell and gene therapies. This shift places the industry not just within hospital settings and blood centers, but squarely within the pharmaceutical and biotechnology manufacturing domain. Key stakeholders now include Chief Scientific Officers (CSOs) and Chief Manufacturing Officers (CMOs) who require not just devices, but integrated, validated, closed-system solutions for compliant cell isolation, driving demand for innovative, high-purity systems.
Explore the company's market share breakdown :
Quantitative Growth Drivers and Market Velocity
The market's velocity is underpinned by a secular trend towards personalized medicine and high-value therapeutic modalities.
- Growth Breakdown & 2030 Projections:
- 2024 Valuation: $2.138 Billion (Estimated)
- Projected 2035 Valuation: $4.651 Billion
- Forecasted CAGR (2025–2035): 7.32%
- Segment Outperformance: Devices (Centrifugation-based): Projected 10.85% CAGR through 2030.
- Segment Outperformance: Research Application: Projected 12.10% CAGR through 2030.
Primary Catalysts
- CAR-T Therapy Commercialization and Pipeline Expansion: The most significant growth catalyst is the regulatory approval and commercial rollout of CAR-T and other T-cell-based immunotherapies. Leukapheresis is the obligatory starting point, supplying the raw material (autologous T-cells). Each commercially approved cell therapy requires a constant, high-volume supply of apheresis procedures, guaranteeing a continuous revenue stream for device and disposable manufacturers. The increasing number of Phase III and Phase IV trials globally locks in future demand.
- Technological Shift to Automated, Closed Systems: Next-generation devices, featuring continuous-flow centrifugation and optical sensors, significantly enhance cell yield and purity. This innovation directly addresses the critical constraint in cell manufacturing: consistency and quality of the starting material. The move toward integrated, closed-loop systems also reduces contamination risk, streamlines operations, and lowers the need for extensive manual processing, accelerating adoption in high-throughput manufacturing sites.
- Surging Demand for Research-Grade Leukopaks: Pharmaceutical and biotech companies require substantial volumes of fresh and cryopreserved leukopaks to develop and optimize new cell and gene therapies. This rising demand in the research application segment—projected to grow at a 12.10% CAGR—is a distinct and high-value driver, often involving specialized collection protocols that generate superior margins for suppliers of both the collection devices and the biological material itself.
Strategic Hurdles
- High Cost and Reimbursement Complexity: The high initial capital cost of advanced leukapheresis devices, coupled with the expensive nature of therapeutic leukapheresis procedures and the subsequent cell therapies, creates a significant adoption restraint, especially in emerging markets. Unfavorable or inconsistent reimbursement policies across different national healthcare systems remain a critical challenge to widespread market penetration.
- Shortage of Skilled Apheresis Professionals: The specialized nature of therapeutic and high-purity leukapheresis requires highly trained clinical staff. A global shortage of qualified apheresis nurses and technologists directly limits the volume of procedures that can be performed, acting as a functional ceiling on market growth, particularly in regional hubs experiencing rapid expansion.
Segment-Level Analysis: Demand Patterns and Opportunities
The market segmentation reveals a clear trajectory favoring devices and applications linked to personalized medicine.
- By Product Type:
- The Disposables segment currently holds the largest market valuation share due to their recurrent, high-volume consumption per procedure, reinforcing predictable cash flows for manufacturers.
- The Leukapheresis Devices segment is poised to significantly outperform the general market CAGR with a projected growth rate of 10.85% through 2030, driven by the replacement cycle of older equipment and the adoption of cutting-edge automated systems by newly established cell therapy centers.
- By Application:
- Research Application is forecast to be the fastest-growing application segment, expanding at a compelling 12.10% CAGR. This accelerated growth is primarily fueled by the sheer volume of cell line development, vector optimization, and preclinical testing required by the massive global investment in immunotherapy research.
- Therapeutic Application remains dominant in terms of current procedural volume, especially for managing conditions like hyperleukocytosis and for stem cell mobilization, ensuring a stable, foundational revenue stream.
Competitive Intelligence and Industry Consolidation
The competitive landscape is characterized by the dominance of a few established global players in the apheresis equipment space, alongside a rapidly growing ecosystem of specialized cell product providers. Competitive benchmarking indicates that success is shifting from manufacturing scale to integration capability.
The top five-to-seven market leaders—including Terumo BCT, Haemonetics Corporation, Asahi Kasei Medical, and Fresenius Kabi—maintain their strategic positioning through a combination of large installed device bases, proprietary consumable sets, and extensive global service networks. These companies are actively pursuing portfolio diversification through targeted acquisitions in the cell processing space to offer end-to-end solutions. For instance, recent FDA clearances for advanced automated systems from major players underscore a commitment to high-yield, closed-system technology, a crucial factor for CMOs. The mid-tier of the market is witnessing intense M&A activity, primarily driven by biotech firms acquiring niche cell processing or leukopak suppliers (e.g., Charles River Laboratories and Lonza Group's activities), aiming to secure their raw material supply chain and bolster their contract development and manufacturing (CDMO) services. R&D spending is heavily focused on microfluidics, label-free separation, and AI-powered automation to improve cell viability and reduce procedural time.
Regional Dynamics: Identifying High-Growth Hubs
Geographic analysis reveals distinct growth catalysts across major regions, driven by regulatory maturity and investment in biotech infrastructure.
- North America (NA): Remains the largest market by market valuation, primarily due to its advanced healthcare infrastructure, high per-capita healthcare expenditure, and the presence of the world’s leading biopharmaceutical companies and academic research institutions. Favorable regulatory pathways and robust reimbursement for cutting-edge therapies ensure continued market penetration and sustained dominance.
- Europe: Holds a significant share, driven by strong public healthcare systems and focused research programs in countries like Germany and the UK. The implementation of the EU’s Advanced Therapy Medicinal Products (ATMP) regulation is creating a secular trend of standardization, which favors adoption of new, compliant device platforms.
- Asia-Pacific (APAC): Projected to be the fastest-growing region, exhibiting the highest CAGR. This is a demographic-driven opportunity, fueled by rising disposable incomes, improving healthcare access, and significant governmental investment in biotechnology and clinical trial infrastructure, particularly in China, Japan, and India. The rapid adoption of Western-approved cell therapies is driving accelerated demand for Leukapheresis Devices Market infrastructure.
Future Outlook: Navigating the Path to 2030
The trajectory of the Leukapheresis Devices Market is intrinsically tied to the success of advanced cell and gene therapies. The future competitive battlefield will not be based on device count, but on the ability to provide integrated, quality-assured, and scalable solutions for the collection of high-purity leukocytes. Stakeholders who can bridge the gap between clinical apheresis and pharmaceutical manufacturing—offering seamless, validated, and low-variability cell collection processes—are positioned for maximum revenue optimization.
A Winning Strategy for market players involves:
- Aggressive R&D in automation and miniaturization for point-of-care or even home-based leukapheresis.
- Strategic partnerships with leading cell therapy developers (CDMOs/Biotech) to lock in disposable supply contracts.
- Targeted investment in APAC manufacturing and distribution networks to capitalize on the fastest regional CAGR.
The Leukapheresis Devices Market is no longer a peripheral medical device sector; it is a critical enabler of the future of personalized medicine. Continued, aggressive technological innovation will ensure the market surpasses the $4.6 Billion mark well before 2030 projections, cementing its place as a high-growth sector within the global life sciences economy.
Explore the company's market share breakdown :
https://www.databridgemarketresearch.com/reports/global-leukapheresis-devices-market
Browse More Reports:
Global Bariatric Patient Room Market
Global Candelilla Wax Market
Global Confocal Laser Scanning Market
Global Contour and Highlight Market
Global Dairy Enzymes Market
Global Fluvoxamine Market
Global Fracking Fluid and Chemical Market
Global Full Egg Replacer Market
Global Glass Reinforced Plastics (GRP) Pipes Market
Global Hydrogenated C6-14 Olefin Polymers Market
Global Hydrogen Bromide Market
Global Neotame Market
Global Neurogenic Bladder Infections Market
Global Next Generation Packaging Market
Global Planting Equipment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"
Schema-Ready FAQ (PAA Style)
What is the expected growth rate of the Leukapheresis Devices Market?
The global Leukapheresis Devices Market is anticipated to exhibit a resilient Compound Annual Growth Rate (CAGR) of 7.32% over the forecast period of 2025 to 2035, driven primarily by the escalating demand for high-quality leukocyte material required for the rapidly expanding manufacturing needs of CAR-T and other cell-based immunotherapies.
Which segment of the Leukapheresis Devices Market is growing the fastest?
The Research Application segment is projected to record the highest growth velocity, with a forecasted CAGR of 12.10% through 2030. This accelerated growth is directly attributable to the global surge in research and development spending by pharmaceutical and biotechnology companies focused on creating novel cell and gene therapies, which rely on standardized leukopaks.
What is the primary driver of the increase in market valuation?
The primary driver behind the market’s rising market valuation is the commercial success and pipeline maturation of CAR-T cell therapies. As these therapies transition from clinical trials to approved commercial products, they generate a consistent, non-discretionary demand for leukapheresis as the essential first step in the manufacturing process, fundamentally transforming the traditional revenue streams.
Which region holds the largest share in the Leukapheresis Devices Market?
North America currently holds the largest market valuation share in the Leukapheresis Devices Market. This dominance is underpinned by a confluence of factors, including its superior healthcare infrastructure, high patient enrollment in clinical trials, significant capital investment in R&D, and a regulatory environment that facilitates the rapid adoption of advanced medical technologies and therapeutic procedures.
What are the key products comprising the Leukapheresis Devices Market?
The market is broadly segmented into two key product categories: Leukapheresis Devices (comprising automated apheresis systems, cell separators, and columns) and Leukapheresis Disposables (which include single-use kits, tubing sets, and essential leukoreduction filters). The disposables segment generates the highest transactional volume and ensures consistent revenue streams for manufacturers.
What are the major strategic hurdles to greater market penetration?
The principal strategic hurdles include the high acquisition cost of advanced automated Leukapheresis Devices, which limits adoption in capital-constrained facilities, and the pervasive issue of a global shortage of highly trained apheresis professionals. These factors combine to constrain procedural volume and complicate widespread market penetration, particularly in developing economies.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


